Career experiences
Prof. ZHAO Lili was born in Changge, Henan Province of China in 1984. In 2003, she enrolled at Henan University, and became undergraduate at the College of Chemistry and Chemical Engineering majoring in chemistry. She received bachelor degree (in Chemistry) from Henan University in July 2007, and then became a formal graduate student in the University of Chinese Academy of Sciences (GUCAS) in September 2007, majored in Physical Chemistry/Computational Chemistry. She was promoted as a PhD student in 2009, and received her PhD degree in July of 2012. After that, she joined immediately in the institute of high performance computing (IHPC) department of A*star in Singapore in August 2012, where she spent two years doing some basic research project and industry project. During September 2014 ~ October 2016, she was supported by the Alexander von Humboldt fellowship, and work as postdoc in Prof. Gernot Frenking’s group at Marburg University, Germany.
In November 2016, Prof. ZHAO settled as a professor in the Institute of advanced synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University. In 2019, he was appointed as "Distinguished Professor of Jiangsu Province". In 2020, she got the third prize of the 17th Young Teacher Award of Higher Education Institutions by Fok Yingdong Education Foundation. During the last five years, she was supported by the National Natural Science Foundation Youth Program and General Program, Jiangsu Natural Science Foundation Youth Program and Excellent Youth Program, Jiangsu Distinguished Professor Fund Program, etc. She teaches courses “Physical Chemistry” for the undergraduate students and “Quantum Chemistry” for the graduate students. Since 2017, more than 80 papers were published in scientific journals, including Science, Nature Chem., J. Am. Chem. Soc., Angew. Chem., Int. Ed., Nature Commun., ACS Catal., Chem. Sci., Chem. Rev., Nature Rev. Chem. etc.
Exploring the chemistry with computers
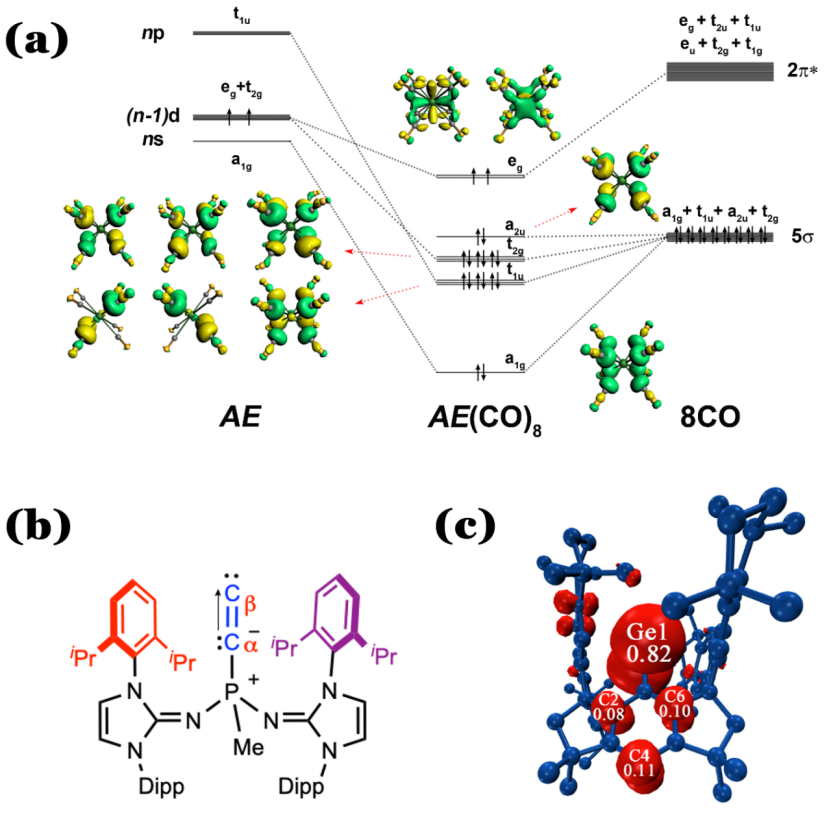
As early as 1921, Langmuir proposed the well-known 2, 8, 18, and 32 electron rules, to understand the structures and stabilities of molecules. Generally, the octet rule for S, P block in the periodic table of elements, the 18-electron rule for d block, and 32-electron rule for f block. The electron rules are the useful guideline to understand the chemical behaviour of the elements. Alkaline earth (AE) elements are a typical main-group atoms, normally obey the Octet electron rule. However, Prof. Zhao found that Ba severes as honorary transition metal in Ba(CO)+ (Angew. Chem., Int. Ed. 2018, 57, 3974-3980), which uses its 5d electrons in chemical bonding formation. Later, her experimental collaborators observed the satble AE(CO)8 comlex at the low-temperature matrix. EDA-NOCV analysis revealed that the AE-CO bonding comes mainly from the [AE(dπ)]→(CO)8 π-backdonation, which explains the large red-shift of the C-O stretching mode. Prof. Zhao and her collaborators further proposed that the Alkaline earth elements (e.g., Ca, Sr, Ba) can obey the 18-electron rule as shown in Figure (a) as below (Science. 2018, 361, 912-916.), which challenged previous notions of limitations in the 18-electron rule and provides complexes exhibiting very interesting bonding motifs in the process.
Prof. Zhao’s team also designed other novel molecules with unusual chemical bonding nature. For example, they designed and isolated a more stable and naked C2 molecule as L→C2 using bulky phosphine ligand bearing two imidazolidin-2-iminato groups (L is (NHCR=N)2(CH3)P) (Nature Chemistry 2021, 13, 89-93.). EDA-NOCV analysis indicated that P–Cα bond has fragments of C2- and the phosphine+ ligand lied toward behaving more like resonance Figure (b) as below. They recently reported the first crystalline heavier carbyne radical analogue (Nature Chemistry 2022, DOI: doi.org/10.1038/s41557-022-01081-1.) the germylyne radical MsFluind*-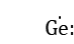 (Figure (c) below), which bears an essentially one-coordinate germanium atom. Our study revealed that the germylyne radical features a doublet ground state, and the three non-bonding valence electrons at the germanium atom.
(Figure (c) below), which bears an essentially one-coordinate germanium atom. Our study revealed that the germylyne radical features a doublet ground state, and the three non-bonding valence electrons at the germanium atom.